This heterogeneous group of conditions is associated with monoclonal immunoglobulin in serum or urine, and is characterized by disordered proliferation of monoclonal lymphocytes or plasma cells. The clinical phenotypes of these conditions are determined by the rate of accumulation, site and biological properties of both the ab- normal cells and the monoclonal protein.
Showing posts with label HEMATOLOGY. Show all posts
Showing posts with label HEMATOLOGY. Show all posts
Tuesday, January 20, 2015
Monday, January 19, 2015
The Myelodysplastic Syndromes
Introduction
• The myelodysplastic syndromes (MDS) are a group of clonal haemopoietic disorders. They are characterized by:
• Ineffective haemopoiesis resulting in peripheral blood cytope- nias of all three lineages, but especially anaemia
• Increased risk (30%) of transformation to acute myeloid leukaemia
• MDS is mainly a disease of the elderly, with a median age at diag- nosis of 60–75 years. It does, however, affect younger adults also. MDS is rare in children, and is associated with genetic disorders such as Fanconi’s anaemia.
Tuesday, January 13, 2015
Platelet Disorders
Platelets are small anucleate cells produced predominantly by the bone marrow megakaryocytes as a result of budding of the cytoplasmic membrane. Megakaryocytes are derived from the haemopoietic stem cell, which is stimulated to differentiate to mature megakaryocytes under the influence of various cytokines, including thrombopoietin. Platelets play a key role in securing primary haemostasis.
Once released from the bone marrow, young platelets are trapped in the spleen for up to 36 hours before entering the circulation, where they have a primary haemostatic role. Their normal lifespan is 7–10 days and the normal platelet count for all age groups is 150–450 × 10^9/L. The mean platelet diameter is 1–2 µm, and the normal range for cell volume (mean platelet volume; MPV) is 8–11 fL. Although platelets are non-nucleated cells, those that have recently been released from the bone marrow contain RNA and are known as reticulated platelets. They normally represent 8–16% of the total count and they indirectly indicate the state of marrow production.
Monday, January 12, 2015
The Acute Leukaemias
Acute leukaemia is a malignant disorder of white cells caused by a failure of normal differentiation of haemopoietic stem cells and pro- genitors into mature cells. This results in the accumulation of primitive leukaemic cells within the bone marrow cavity, causing bone marrow failure, and as a consequence patients typically present with anaemia, thrombocytopenia or neutropenia (Box 6.1).
Much progress has been made in understanding the pathogenesis of the acute leukaemias, and it is now clear that they occur because of the acquisition of distinct genetic abnormalities in haemopoietic stem cells or committed progenitors. These molecular abnormalities frequently occur as the result of chromosomal translocations or the loss of chromosomal material. In addition, activating mutations in genes regulating cellular proliferation, such as tyrosine kinase genes, are commonly identified. Malignant transformation of primitive cells with the capacity to develop into cells of the myeloid lineage results in acute myeloid leukaemia (AML), while acquired genetic
Wednesday, December 31, 2014
Chronic Myeloid Leukaemia
Chronic myeloid leukaemia (CML) is a clonal malignant myeloproliferative disorder believed to originate in a single abnormal haemopoietic stem cell. The progeny of this abnormal stem cell proliferate over months or years, so that, by the time the leukaemia is diagnosed, the bone marrow is grossly hypercellular and the number of leucocytes is greatly increased in the peripheral blood. Normal blood cell production is almost completely replaced by leukaemia cells, which, however, still function almost normally.
Tuesday, December 30, 2014
Polycythaemia, Essential Thrombocythaemia and Myelofibrosis
Polycythaemia vera (PV), essential thrombocythaemia (ET) and idiopathic myelofibrosis (IMF), known collectively as the classic myeloproliferative disorders (MPDs), are clonal disorders originating from a neoplastic haemopoietic stem cell. They are most common in middle or older age, and share several features, including a potential to transform into acute leukaemia and into each other. Treatment of PV and ET can greatly influence prognosis, hence the importance
The Hereditary Anaemias
Hereditary anaemias include disorders of the structure or synthesis of haemoglobin (Hb), deficiencies of enzymes that provide the red cell with energy or protect it from chemical damage and abnormalities of the proteins of the red cell’s membrane. Inherited diseases of haemoglobin (haemoglobinopathies) are by far the most important. The structure of human Hb changes during development (Fig.3.1). By the 12th week of gestation, embryonic haemoglobin is replaced by fetal haemoglobin (Hb F), which is slowly replaced after birth by the adult haemoglobins, Hb A and Hb A2. Each type of haemoglobin consists of two different pairs of peptide chains; Hb A has the structure α2β2 (namely, two α chains plus two β chains), Hb A2 has the structure α 2δ 2 and Hb F, α 2γ2.
Macrocytic Anaemias
Macrocytosis is a rise in the mean cell volume (MCV) of red cells above the normal range (in adults 80–95 fl). It is detected using a blood count, in which the MCV and other red cell indices are meas- ured. The MCV is lower in children than in adults, with a normal mean of 70 fl at 1 year of age, rising by about 1 fl each year until it reaches the adult volume at puberty.
The causes of macrocytosis fall into two groups: (i) deficiency of vitamin B12 (cobalamin) or folate (or rarely abnormalities of their metabolism), in which the bone marrow is megaloblastic (Box 2.1) and (ii) other causes (Box 2.2), in which the bone marrow is usually normoblastic. In this chapter, the two groups are considered sepa- rately. The steps to diagnose the cause of macrocytosis and subse- quently to manage it are then considered.
Iron Deficiency Anaemia
OVERVIEW
• Iron deficiency is the commonest cause of anaemia worldwide
• Iron deficiency is usually easily diagnosed from the red cell indices
• A drop in haemoglobin is generally a late feature of iron deficiency
• The serum ferritin is a reliable means of confirming the diagnosis but may be falsely normal or even elevated as a reactive phenomenon as ferritin is an acute phase protein
• Iron deficiency is not a diagnosis in itself and in males and postmenopasual women blood loss from the gastrointestinal tract must be excluded
• Oral iron is preferred for iron replacement therapy, but occasionally parenteral iron is required
Iron deficiency is the commonest cause of anaemia worldwide and is frequently seen in general practice. Iron deficiency anaemia is caused by defective synthesis of haemoglobin, resulting in red cells that are smaller than normal (microcytic) and contain reduced amounts of haemoglobin (hypochromic).
Iron metabolism
Iron has a pivotal role in many metabolic processes, and the average adult contains 3–5 g of iron, of which two-thirds is in the oxygen- carrying molecule haemoglobin.
A normal Western diet provides about 15 mg of iron daily, of which 5–10% is absorbed (~ 1 mg), principally in the duodenum and upper jejunum, where the acidic conditions help the absorption of iron in the ferrous form. Absorption is helped by the presence of other re- ducing substances, such as hydrochloric acid and ascorbic acid. The body has the capacity to increase its iron absorption in the face of in- creased demand, for example, in pregnancy, lactation, growth spurts and iron deficiency (Box 1.1).
Once absorbed from the bowel, iron is transported across the mucosal cell to the blood, where it is carried by the protein transferrin to developing red cells in the bone marrow. Iron stores comprise fer- ritin, a labile and readily accessible source of iron and haemosiderin, an insoluble form found predominantly in macrophages.
About 1 mg of iron a day is shed from the body in urine, faeces, sweat
Haematological Findings in Health and Disease
The blood film and count in healthy individuals
The microscopic features of normal blood cells and the normal range for the blood count have been discussed in Chapter 1. In assessing what is ‘normal’ it is necessary to consider the gender, age and ethnic origin of the person being investigated.
Gender
Adult men have a higher normal range for RBC, Hb and PCV/Hct than adult women but women tend to have a somewhat higher WBC and platelet count (see Table 1.2).
Neonates, infants, and children
The blood counts of healthy neonates, infants and children differ greatly from those of healthy adults (see Table 1.3). Neonates have a higher Hb, MCV, WBC, neutrophil count and lymphocyte count than adults. Children in general have a higher lymphocyte count than adults. They tend to have a slightly lower Hb and MCV.
Pregnancy
Physiological variation in the blood count occurs during preg- nancy. The Hb falls, the MCV rises slightly and the WBC and neutrophil count rise. Immature cells (myelocytes and occasion-al promyelocytes) appear in the blood and there may be ‘toxic’granulation and Döhle bodies.
Ethnic variation
The blood counts of healthy Africans and Afro-Caribbeans (see Table 1.3) often show a lower white cell and neutrophil count than is usual in Caucasians (see Table 1.2). There is also a tendency to a lower platelet count, particularly in Africans.
Abnormalities of red cells
Polycythaemia
Polycythaemia is an increase in the Hb. It is usually accompa- nied by an increase in the RBC and PCV/Hct. It can be caused by a true increase in the total volume of red cells in the circulation (true polycythaemia) or by a decrease in the total plasma volume (apparent or relative or pseudo-polycythaemia). It is not possible to distinguish true from apparent polycythaemia by a blood film or count. True polycythaemia is caused by overproduction of red cells. Normally red cell production is driven by erythropoietin production in response to a diminished oxygen supply to the kidney. Overproduction of red cells may be an erythropoietin- mediated physiological response to hypoxia or it may be caused by inappropriate secretion of erythropoietin or by mechanisms independent of erythropoietin. Some of the important causes of polycythaemia, classified according to mechanism, are shown in Table 4.1.
Polycythaemia vera
Polycythaemia vera, also referred to as polycythaemia rubra vera or primary proliferative polycythaemia, is a myeloproliferative disorder characterized by overproduction of red cells. In many patients there is also overproduction of white cells and platelets. Clinical features are facial plethora and sometimes a moderate degree of splenomegaly. The disease may be complicated by arterial thrombosis and peripheral ischaemia.
 |
| Table 4.1 Some important causes of polycythaemia, classified according to mechanism. |
Tuesday, December 23, 2014
Assessing White Cells and Platelets
White cells and platelets may be increased or decreased in number. They may also show morphological abnormalities, either inherited or acquired. Assessing whether the numbers of individual types of white cell are increased or decreased requires a differential count. However, the differential count is of little importance in itself and should only be used to calculate the absolute numbers of each cell type. The absolute counts are then compared with those expected in healthy people of the same age, sex and ethnic group. The terms used in describing numerical abnormalities in white cells and platelets are defined in Table 3.1.
 |
| Table 3.1 Terminology used for abnormalities of white cell and platelet numbers |
Assessing Red Cells
Red cells should be assessed as to their:
• number
• size
• shape
• degree of haemoglobinization
• distribution in the blood film.
Their appearance should be described using a standard terminology.
Assessing red cell number and distribution (anaemia, polycythaemia, rouleaux formation, red cell agglutination)
The thickness of a film of blood spread on a glass slide is deter- mined by how thick the blood is, i.e. by its viscosity. This in turn is determined by the Hb. In a normal blood film it is possible to find a part of the film which is ideal for microscopic examination where the red cells are touching but not overlapping. If the Hb is abnormally high (a condition referred to as polycythaemia) the blood has a high viscosity and the film of blood on the glass slide is thick. The red cells therefore appear packed together through- out the whole length of the film. The term ‘packed film’ is often used. Conversely, when a patient is anaemic the viscosity of the blood is low, the blood film is very thin and there are large spaces between the red cells. The effect of Hb on the blood film can be seen by comparing Figs 2.1, 2.2 and 2.3.
Usually red cells are distributed fairly regularly on the slide. Two abnormalities of distribution may occur. When there is an
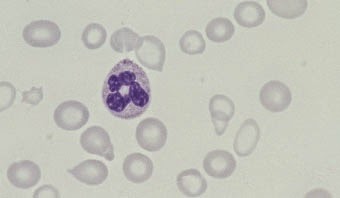 |
Fig. 2.1 Anaemia (caused
by iron deficiency).
|
Monday, December 22, 2014
The Blood Film and Count (Part 2)
Peripheral blood cells are produced in the bone marrow. Their precursors are referred to as haemopoietic cells (Fig. 1.12). The only significant function of haemopoietic cells is the production of mature end cells. Recognizable haemopoietic precursors are present in the circulating blood of healthy subjects but, except in the neonatal period and during pregnancy, they are quite uncom- mon and are not often noted in a blood film. They are much commoner in patients with leukaemia or other haematological disorders and in patients with severe infection or other serious systemic diseases.
Myeloblasts (Fig. 1.13) are very rare in the blood of healthy subjects. They are larger than lymphocytes but often smaller than monocytes. They have a high nucleocytoplasmic ratio and scanty to moderate amounts of cytoplasm, which varies from weakly to moderately basophilic. (Basophilic in this context in- dicates a blue colour consequent on the uptake of basic dyes.) The nucleus is approximately round, nuclear chromatin is dif- fuse and nucleoli may be apparent. In patients with leukaemia and related disorders, the cytoplasm may contain small numbers of azurophilic granules or other inclusions or vacuoles (see page 91). Myeloblasts are precursors of neutrophils, eosinophils and basophils.
Promyelocytes
Promyelocytes (Fig. 1.14) are rare in the blood of healthy people. They are larger than myeloblasts with more plentiful cytoplasm and consequently a lower nucleocytoplasmic ratio. The cyto- plasm is more basophilic than that of a myeloblast and contains azurophilic (pinkish-purple) primary granules. Sometimes there is a more lightly staining zone in the cytoplasm adjacent to the nucleus, which represents the Golgi apparatus, where granules are produced. The nucleus is round or oval, is usually eccentric, shows some chromatin condensation and has a
Friday, December 12, 2014
The Blood Film and Count (Part 1)
Blood
Blood is a life-sustaining fluid which circulates through the heart and blood vessels. It carries oxygen and nutrients to the tissues and waste products to the lungs, liver and kidneys, where they can be removed from the body. Usually when blood is removed from the body it forms a solid blood clot. However, if clotting is prevented by mixing with an anticoagulant, the blood separates, under the influence of gravity, into three layers (Fig. 1.1). The bottom layer is deep red in colour and is composed of red cells. The top layer is clear and pale yellow. It is called plasma and is composed of various salts and proteins dissolved in water. In between is a narrow layer called the buffy coat because of its buff or yellowish white colour. The buffy coat is composed mainly of cells of a variety of types, collectively known as white cells. In addition there are small cellular fragments, called platelets, which have a role in blood clotting.
The blood film
Although we can judge the proportions of red cells and white cells in a tube of sedimented blood, we get far more information if the blood is carefully mixed and a thin layer is spread on a glass slide to form a blood film. The blood cells are then preserved by exposure to the alcohol methanol, a process known as fixation. The fixed film of blood is stained with a mixture of several dyes so that the individual cells can be recognized when they are examined with a microscope. After staining, the colour of red
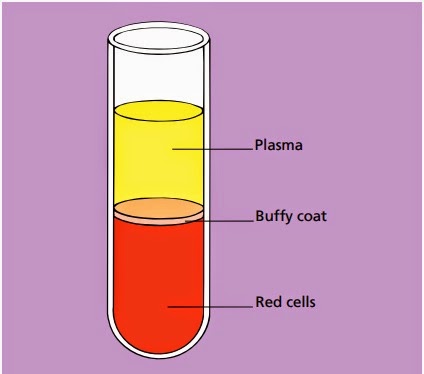 |
Fig. 1.1Diagram of a tube of anticoagulated blood which has
been allowed to sediment,
showing the separation of blood into red cells, a buffy coat (white cells and platelets) and
plasma.
|
Subscribe to:
Comments (Atom)










