OVERVIEW
• Iron deficiency is the commonest cause of anaemia worldwide
• Iron deficiency is usually easily diagnosed from the red cell indices
• A drop in haemoglobin is generally a late feature of iron deficiency
• The serum ferritin is a reliable means of confirming the diagnosis but may be falsely normal or even elevated as a reactive phenomenon as ferritin is an acute phase protein
• Iron deficiency is not a diagnosis in itself and in males and postmenopasual women blood loss from the gastrointestinal tract must be excluded
• Oral iron is preferred for iron replacement therapy, but occasionally parenteral iron is required
Iron deficiency is the commonest cause of anaemia worldwide and is frequently seen in general practice. Iron deficiency anaemia is caused by defective synthesis of haemoglobin, resulting in red cells that are smaller than normal (microcytic) and contain reduced amounts of haemoglobin (hypochromic).
Iron metabolism
Iron has a pivotal role in many metabolic processes, and the average adult contains 3–5 g of iron, of which two-thirds is in the oxygen- carrying molecule haemoglobin.
A normal Western diet provides about 15 mg of iron daily, of which 5–10% is absorbed (~ 1 mg), principally in the duodenum and upper jejunum, where the acidic conditions help the absorption of iron in the ferrous form. Absorption is helped by the presence of other re- ducing substances, such as hydrochloric acid and ascorbic acid. The body has the capacity to increase its iron absorption in the face of in- creased demand, for example, in pregnancy, lactation, growth spurts and iron deficiency (Box 1.1).
Once absorbed from the bowel, iron is transported across the mucosal cell to the blood, where it is carried by the protein transferrin to developing red cells in the bone marrow. Iron stores comprise fer- ritin, a labile and readily accessible source of iron and haemosiderin, an insoluble form found predominantly in macrophages.
About 1 mg of iron a day is shed from the body in urine, faeces, sweat
and cells shed from the skin and gastrointestinal tract. Men- strual losses of an additional 20 mg per month and the increased requirements of pregnancy (500–1000 mg) contribute to the higher incidence of iron deficiency in women of reproductive age (Table 1.1, Box 1.2).
Clinical features of iron deficiency
The symptoms accompanying iron deficiency depend on how rapidly the anaemia develops. In cases of chronic, slow blood loss, the body adapts to the increasing anaemia and patients can often tolerate ex- tremely low concentrations of haemoglobin, for example, < 7.0 g/dL, with remarkably few symptoms. Most patients complain of increasing lethargy and dyspnoea. More unusual symptoms are headaches, tin- nitus and taste disturbance.
 |
| Table 1.1 Daily dietary iron requirements |
On examination, several skin, nail and other epithelial changes may be seen in chronic iron deficiency. Atrophy of the skin occurs in about a third of patients and (rarely nowadays) nail changes such as koilo- nychia (spoon-shaped nails; Fig. 1.1) may result in brittle, flattened nails. Patients may also complain of angular stomatitis, in which pain- ful cracks appear at the angle of the mouth, sometimes accompanied by glossitis. Although uncommon, oesophageal and pharyngeal webs can be a feature of iron deficiency anaemia (consider this in middle aged women presenting with dysphagia). These changes are believed
 |
Figure 1.1
Nail changes
in
iron deficiency anaemia (koilonychia).
|
 |
| Figure 1.2 Diagnosis and investigation of iron deficiency anaemia. |
to be due to a reduction in the iron-containing enzymes in the epi- thelium and gastrointestinal tract. Few of these epithelial changes are seen in modern practice, and they are of limited diagnostic value.
Tachycardia and cardiac failure may occur with severe anaemia irrespective of cause and, in such cases, prompt remedial action should be taken.
When iron deficiency is confirmed, a full clinical history, includ- ing leading questions on possible gastrointestinal blood loss or mal- absorption (as in, for example, coeliac disease), should be obtained. Menstrual losses should be assessed and the importance of dietary factors and regular blood donation should not be overlooked (Fig. 1.2). Diet alone is seldom the sole cause of iron deficiency anaemia in the UK except when it prevents an adequate response to a physi- ological challenge, as in pregnancy, for example.
Laboratory investigations
A full blood count and film should be assessed (Box 1.3). These will confirm the anaemia; recognizing the indices of iron deficiency is usually straightforward (reduced haemoglobin concentration, re- duced mean cell volume, reduced mean cell haemoglobin, reduced mean cell haemoglobin concentration) (Table 1.2). Some modern analysers will determine the percentage of hypochromic red cells, which may be high before the anaemia develops (it is worth not- ing that a reduction in haemoglobin concentration is a late feature of iron deficiency). The blood film shows microcytic hypochromic red cells (Fig. 1.3). Hypochromic anaemia occurs in other disorders, such as anaemia of chronic disorders and sideroblastic anaemias,
and in globin synthesis disorders, such as thalassaemia (Table 1.3). To help to differentiate the type, further haematinic assays may be necessary. Historically, serum iron and total iron binding capacity (TIBC) were used in the diagnosis of iron deficiency anaemia, but because of the wide diurnal variation seen in iron levels and the lack of sensitivity, these assays are seldom used today. Difficulties in diagnosis arise when more than one type of anaemia is present, for example, iron deficiency and folate deficiency in malabsorption, in a population where thalassaemia is present, or in pregnancy, when the interpretation of red cell indices may be difficult.
Haematinic assays will demonstrate reduced serum ferritin con- centration in straightforward iron deficiency. As an acute phase pro- tein, however, the serum ferritin concentration may be normal or even raised in inflammatory or malignant disease.
 |
| Figure 1.3 Blood film showing changes of iron deficiency anaemia. |
A prime example of this is found in rheumatoid disease, in which active disease may result in a spuriously raised serum ferritin concen- tration masking an underlying iron deficiency caused by gastrointes- tinal bleeding after non-steroidal analgesic treatment. There may also be confusion in liver disease, as the liver contains stores of ferritin that are released after hepatocellular damage, leading to raised serum ferritin concentrations. In cases where ferritin estimation is likely to be misleading, the soluble transferrin receptor (sTfR) assay may aid the diagnosis. Transferrin receptors are found on the surface of red cells in greater numbers in iron deficiency; a proportion of receptors is shed into the plasma and can be measured using commercial kits. Unlike serum ferritin, the level of sTfR does not rise in inflammatory disorders, and
 |
| Table 1.2 Diagnosis of iron deficiency anaemia |
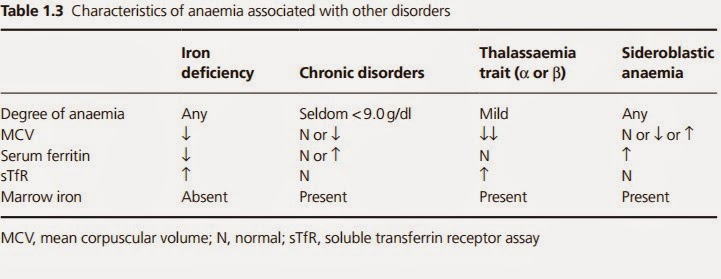 |
| Table 1.3 Characteristics of anaemia associated with other disorders |
hence can help to differentiate between anaemia due to inflamma- tion and iron deficiency.
Diagnostic bone marrow sampling is seldom performed in simple iron deficiency, but, if the diagnosis is in doubt, a marrow aspirate may be carried out to demonstrate absent bone marrow stores.
When iron deficiency has been diagnosed, the underlying cause should be investigated and treated. Often the history will indicate the likely source of bleeding, for example, menstrual blood loss or gastrointestinal bleeding. If there is no obvious cause, further in- vestigation generally depends on the age and sex of the patient. In male patients and postmenopausal women, possible gastrointesti- nal blood loss is investigated by visualization of the gastrointestinal tract (endoscopic or barium studies). Faecal occult blood tests are of no value in the investigation of iron deficiency.
Management
Effective management of iron deficiency relies on (i) the appropriate management of the underlying cause (for example, gastrointestinal or menstrual blood loss) and (ii) iron replacement therapy.
Oral iron replacement therapy, with gradual replenishment of iron stores and restoration of haemoglobin, is the preferred treatment. Oral ferrous salts are the treatment of choice (ferric salts are less well absorbed) and usually take the form of ferrous sulphate 200 mg three times daily (providing 65 mg × 3 = 195 mg elemental iron/day) (Fig. 1.4). Alternative preparations include ferrous gluconate and ferrous fumarate (Table 1.4). All three compounds, however, are associated with a high incidence of side effects, including nausea, constipation and diarrhoea. These side effects may be reduced by taking the tablets after meals, but even milder symptoms account for poor compliance with oral iron supplementation. It is worth noting that these lower gastrointestinal symptoms are not dose related. Modified release preparations have been developed to reduce side effects, but in prac- tice prove expensive and often release the iron beyond the sites of optimal absorption.
 |
| Figure 1.4 Oral iron replacement therapy. |
 |
| Table 1.4 Elemental iron content of various oral iron preparations |
Effective iron replacement therapy should result in a rise in hae- moglobin concentration of around 0.1 g/dL per day (about 2 g/dL every 3 weeks), but this varies from patient to patient. Once the hae- moglobin concentration is within the normal range, iron replace- ment should continue for 3 months to replenish the iron stores.
Failure to respond to oral iron therapy
The main reason for failure to respond to oral iron therapy is poor compliance. However, if the losses (for example, bleeding) exceed the amount of iron absorbed daily, the haemoglobin concentration will not rise as expected; this will also be the case in combined de- ficiency states.
The presence of underlying inflammation or malignancy may also lead to a poor response to therapy. Occasionally, malabsorption of iron, such as that seen in coeliac disease, may lead to a failure to respond. Finally, an incorrect diagnosis of iron deficiency anaemia should be considered in patients who fail to respond adequately to iron replacement therapy.
Intravenous and intramuscular iron preparations
Parenteral iron may be used when the patient cannot tolerate oral supplements, for example, when patients have severe gastrointestinal side effects or if the losses exceed the daily amount that can be ab- sorbed orally (Box 1.4). The rise in haemoglobin concentration is no faster with parenteral iron preparations than with oral iron therapy. Intramuscular iron sorbitol (a complex of iron, sorbitol and citric acid) injection was used as a parenteral iron replacement for many years, but was discontinued in the UK in 2003. Generally, around
10–20 deep intramuscular injections were given over 2–3·weeks. However, side effects were common and included pain, skin stain- ing at the site of injection and arthralgia. Newer intravenous iron preparations include iron hydroxide sucrose (Venofer®) and iron dextran (Cosmofer ®, may also be given IM) for use in selected cases and under strict medical supervision, for example, on a haematol- ogy day unit (risk of anaphylaxis or other reactions).
Alternative treatments
Blood transfusion is not indicated unless the patient has decompen- sated due to a drop in haemoglobin concentration and needs a more rapid rise in haemoglobin, for example, in cases of worsening angina or severe coexisting pulmonary disease. In cases of iron deficiency with serious ongoing acute bleeding, blood transfusion may be required.
Prevention When absorption from the diet is likely to be matched or exceeded by losses, extra sources of iron should be considered, for example





No comments:
Post a Comment