Potassium disorders are commonly encountered in clinical practice. They are important because of the role potassium plays in determining the resting membrane potential of cells. Changes in plasma potassium mean that ‘excitable’ cells, such as nerve and muscle, may respond differently to stimuli. In the heart (which is largely muscle and nerve), the consequences can be fatal, e.g. arrhythmias.
Serum potassium and potassium balance
Serum potassium concentration is normally kept within a tight range (3.5–5.3 mmol/ L). Potassium intake is variable (30–100 mmol/day in the U K) and potassium losses (through the kidneys) usually mirror intake. The two most important factors that determine potassium excretion are the glomerular filtration rate and the plasma potassium concentration. A small amount (~5 mmol/day) is lost in the gut. Potassium balance can be disturbed if any of these fluxes is altered (Fig 11.1). An additional factor often implicated in hyperkalaemia and hypokalaemia is redistribution of potassium. Nearly all of the total body potassium (98%) is inside cells. If, for example, there is significant tissue damage, the contents of cells, including potassium, leak out into the extracellular compartment, causing potentially dangerous increases in serum potassium (see below).
Hyperkalaemia
Hyperkalaemia is one of the commonest electrolyte emergencies encountered in clinical practice. If severe (>7.0 mmol/L), it is immediately life-threatening and must be dealt with as an absolute priority; cardiac arrest may be the first mani- festation. ECG changes seen in hyperkalaemia (Fig 11.2) include the classic tall ‘tented’ T-waves and widening of the QRS complex, reflecting altered myocardial contractility. Other symptoms include muscle weakness and paraesthesiae, again reflecting involvement of nerves and muscles.
Hyperkalaemia can be categorized as due to increased intake, redistribution or decreased excretion.
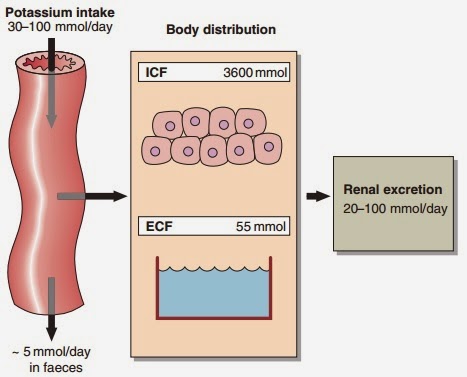 |
| Fig 11.1 Potassium balance |
 |
Fig 11.2 Typical ECG changes associated with hyperkalaemia. (a) Normal ECG
(lead II). (b)
Patient with hyperkalaemia: note peaked T-wave and widening of the QRS complex. |
Decreased excretion
In practice, virtually all patients with hyperkalaemia will have a reduced GFR.
- Renal failure. The kidneys may not be able to excrete a potassium load when the glomerular filtration rate is very low, and hyperkalaemia is a central feature of reduced glomerular function. It is exacerbated by the associated metabolic acidosis, due to the accumulation of organic ions that would normally be excreted.
- Hypoaldosteronism. Aldosterone stimulates sodium reabsorption in the renal tubules at the expense of potassium and hydrogen. This mineralocorticoid activity is shared by many steroid molecules. Deficiency, antagonism or resistance results in loss of sodium, causing a decreased GFR with associated retention of potassium and hydrogen ions. In clinical practice, hyperkalaemia due to hypoaldosteronism is most often seen with the use of angiotensinconverting enzyme (AC E) inhibitors and angiotensin receptor blockers (AR Bs) to treat hypertension; spironolactone and other potassiu-sparing diuretics also antagonize the effect of aldosterone. Less frequently, adrenal insufficiency is responsible .
Figure 11.4 describes an approach to the evaluation of hyperkalaemia.
Redistribution out of cells
- Potassium release from damaged cells. The potassium concentration inside cells (~140 mmol/ L) means that cell damage can give rise to marked hyperkalaemia. This occurs in rhabdomyolysis (where skeletal muscle is broken down), extensive trauma, or rarely tumour lysis syndrome, where malignant cells break down.
 |
| Fig 11.3 Hyperkalaemia is associated with acidosis |
 |
| Fig 11.4 The evaluation of hyperkalaemia |
- Metabolic acidosis. There is a reciprocal relationship between potassium and hydrogen ions. As the concentration of hydrogen ions increases with the development of metabolic acidosis, so potassium ions inside cells are displaced from the cell by hydrogen ions in order to maintain electrochemical neutrality (Fig 11. 3). These hydrogen ion changes cause marked alterations in serum potassium.
- Insulin deficiency. Insulin stimulates cellular uptake of potassium, and plays a central role in treatment of severe hyperkalaemia. Where there is insulin deficiency or severe resistance to the actions of insulin, as in diabetic ketoacidosis, hyperkalaemia is an associated feature.
- Pseudohyperkalaemia. This should be considered when the cause of hyperkalaemia is not readily apparent. Indeed, it is important largely because it can lead to diagnostic dilemmas. It is dealt with in detail below.
- Hyperkalaemic periodic paralysis. This is a rare familial disorder with autosomal dominant inheritance. It presents typically as recurrent attacks of muscle weakness or paralysis, often precipitated by rest after exercise.
Increased intake
Failure to appreciate sources of potassium intake may result in dangerous hyperkalaemia, particularly in patients with impaired renal function. For example, many oral drugs are administered as potassium salts. Potassium may also be given intravenously. Intravenous potassium should not be given faster than 20 mmol/hour except in extreme cases. Occasionally, blood products may give rise to hyperkalaemia (stored red blood cells release potassium down its concentration gradient). The risk of this is reduced by using relatively fresh blood (less than 5 days old) and/or by ‘washing’ units prior to transfusing.
Treatment
- Calcium – usually in the form of calcium gluconate or calcium chloride should be given to counteract the effects of hyperkalaemia on the resting membrane potential of cells.
- Insulin and glucose should be given to promote the uptake of potassium by muscule tissues.
- The underlying cause of the reduction in GFR should be sought and corrected when possible. If the GFR cannot be restored the patient will need to be dialysed. Units treating acutely ill patients will have a written local protocol that should be followed.
Cation exchange resins are not suitable for the treatment of severe hyperkalaemia. They are only useful in the treatment of modest slow increases in potassium.
Pseudohyperkalaemia
This refers to an increase in the concentration of potassium due to its movement out of cells during or after venesection. The commonest causes are: (1) Delay in centrifugation separating plasma/serum from the cells/clot, especially if the specimen is chilled. This is very common in specimens from primary care. (2) In-vitro haemolysis. (3) An increase in the platelet and / or white cell count.
Spurious hyperkalaemia due to haemolysis is usually detected by current laboratory instrumentation or by visible inspection by laboratory staff. The lysis of white cells and/or platelets will not be detected by instrumentation or by inspection.
Formal investigation of suspected pseudohyperkalaemia should include simultaneous collection and processing of serum and plasma specimens (the anticoagulant in plasma specimens prevents clotting). Varying the time of sample centrifugation may also provide evidence, in the form of a progressive steep rise in serum potassium seen with delayed centrifugation.
Clinical note
Some oral drugs are administered as potassium salts. Unexplained, persistent hyperkalaemia should always prompt review of the drug history.
Hyperkalaemia
- Most potassium in the body is intracellular.
- The commonest cause of hyperkalaemia is renal impairment.
- Severe hyperkalaemia is immediately life-threatening and death may occur with no clinical warning signs.
- Sometimes hyperkalaemia is artefactual – pseudohyperkalaemia.

No comments:
Post a Comment